By Dr Anthony Hobson PhD and Jordan Haworth BSc (Functional Gut Clinic. London, UK)
The distinction between gastroparesis and functional dyspepsia (FD) is altogether ambiguous, specifically, both entities manifest with similar upper gastrointestinal (GI) symptoms, including early satiety, epigastric pain or discomfort, and nausea. FD is a disorder of gut brain interaction (DGBI), previously known as a functional GI disorder, and diagnosis is based on a subjective set of criteria [1], whereas the defining characteristic for gastroparesis is objective evidence of delayed gastric emptying in the absence of structural pathology. However, new data from researchers who followed up 720 patients with gastroparesis and 244 with FD found that at 1-year, gastric emptying had become abnormal in 37% of FD patients whilst it had normalised in 42% of patients with gastroparesis [2]. Moreover, there was no correlation between symptom severity and gastric emptying. Clearly, a large proportion of patients can yo-yo between the two, but this study, as recent editorials suggest [3,4], may propose the final nail in the coffin for gastric emptying tests (at least in those without nausea or vomiting).
Interestingly, the researchers at the National Institute of Diabetes and Digestive and Kidney Diseases/National Institutes of Health Gastroparesis Clinical Research Consortium, who carried out this provocative prospective study, performed gastric biopsies in a subset of patients and 9 obese controls without gastroparesis or functional dyspepsia [2]. Histology showed loss of interstitial cells of Cajal (ICCs) and CD206+ macrophages in both gastroparesis and FD groups compared with controls. ICCs are a specialised network of cells that act as pacemakers throughout the GI tract and are responsible for rhythmic propagation of circular muscle contractions involved in peristalsis [5]. Loss of ICC networks has previously been established in gastroparesis and the absence of ICCs is associated with delayed gastric emptying in diabetics and abnormalities of gastric slow waves. However, ICC depletion as a predictor of symptom burden and treatment outcome has yet to be established [6]. One technique that may help to advance our understanding of the pathophysiology of dyspeptic symptoms and guide management is electrogastrography (EGG).
EGG is a non-invasive measurement of gastric myoelectrical activity (GMA) using cutaneous electrodes placed on the abdomen. Indeed, EGG is not a new technique, but it has been reinvigorated with advances in artificial intelligence software, such as high-resolution and body surface mapping. EGG records the pacemaker activity of the ICCs, which generate slow waves at a frequency of about 3 cycles per minute (cpm). Dysrhythmias occur when 3 cpm activity is altered: bradycardia is defined by 0.5 to 2.3 cpm and tachygastria by 3.8 to 15 cpm. EGG correlates with gastric emptying on scintigraphy in patients with FD and gastroparesis [7]. In addition, abnormal gastric slow-wave rhythms are seen in up to 51% of patients with FD and are correlated with symptom severity, specifically for early satiety and bloating [8]. Patients with FD spent more time in bradygastria and tachygastria when fasted compared to controls. Furthermore, Leahy and colleagues[9] performed EGG in 170 patients with FD, 70 with irritable bowel syndrome (IBS), 20 with gastroesophageal reflux disease (GORD) and 30 asymptomatic controls. EGG was abnormal in 36% of FD patients and 25% of IBS patients with concurrent dyspepsia, but EGG was normal in 93% of asymptomatic controls, 90% of GORD patients, and 92% of IBS patients without dyspepsia. Therefore, abnormal gastric rhythms, as defined on EGG, likely play a role in the pathophysiology of FD.
Recent data has also revealed that EGG may indirectly assess pyloric function. The pylorus modulates the rate of gastric emptying where increased pyloric tone and isolated pyloric pressure waves prevent gastric emptying and promote retention of food. Koch et al[10] performed EGG and found that of 33 patients with normal 3 cpm GMA, 78% had a symptomatic response to pyloric therapy (Botox injection or pyloric balloon dilation) for gastroparesis symptoms. The same group later showed that the increased GMA in the normal 3 cpm range (hypernormal pattern) was reduced following pyloroplasty in line with a reduction in gastric emptying time [11]. These clinical findings represent pyloric outlet obstruction, which may be an idiopathic cause of gastroparesis in the absence of identifiable structural pathology. Idiopathic gastroparesis accounts for 39.4% of gastroparesis cases in the UK [12], but EGG may help to identify a subset of patients with hypernormal 3 cpm GMA who will respond to pyloric intervention. Alternatively, the finding of less normal slow waves (hyponormal) on EGG in the pre and/or post-prandial state is consistent with depleted ICC numbers[13]. ICCs are susceptible to injury from inflammation, medication toxicity and viral infections. In some cases, ICC function may not recover fully or if infection persists, the ICC network may diminish over time. These patients are best managed by dietary intervention, although prokinetics may help to normalise gastric slow wave activity. In a pilot, randomised clinical trial, Ascecio and colleagues[14] showed that metoclopramide and domperidone similarly increased the dominant 3 cpm frequency of gastric pacemaker activity and improved gastric motility by restoring a normogastric pattern on EGG in septic patients. Previously, long-term domperidone treatment has been shown to reduce upper GI symptoms and increase 3 cpm activity in a small sample of patients with diabetic gastroparesis [15]. Another prokinetic agent, erythromycin, significantly increased the 3 cpm frequency in 10 patients with gastroparesis together with an improvement in upper GI symptoms [15].
Typically, prokinetics do not address gastric accommodation, which can be indirectly measured on EGG during the water load satiety test. The patient consumes water in a fasted state until feeling satiated. Ingestion of less than 300 mL is associated with poor gastric accommodation. Buspirone, a 5-hydroxytryptamine 1A receptor agonist, has been shown to significantly increase gastric accommodation versus placebo [16].
Ultimately, gastric emptying studies have limited sensitivity and specificity and poorly predict symptom severity and outcomes in patients with gastroparesis and FD. EGG can identify several subsets of patients with abnormal gastric slow waves and pyloric dysfunction. Combining gastric emptying with EGG could potentially provide greater insight into the pathophysiological mechanisms in symptoms of gastroparesis, and even tailor treatment. This requires standardisation of techniques and robust studies in patients with gastroparesis and FD.
EGG practical steps:
The patient is fasted for at least 6-hours and typically stops prokinetic therapy for at least 3-days before testing, which is akin to standard preparatory protocols for gastric emptying studies. Although, patients can be tested on therapy to determine efficacy if EGG study was previously abnormal. Unfortunately, the protocol for EGG in the current literature is widely heterogenous. In order to pragmatically standardise EGG recordings and to gain maximal interpretable yield, a protocol has been designed that is easily implemented in the clinic and which reduces intra-subject variability as much as possible. The first steps are to gently abrase the skin on the abdominal wall in three positions with the central electrode placed between the xiphisternum and the umbilicus, with the other two electrodes placed approximately 5-10cm either side of this depending on the individual’s anatomy and size.
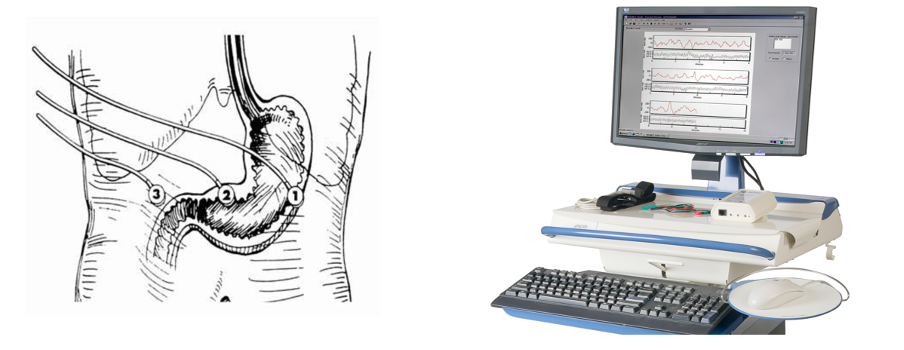
Figure 1 – Shows a diagram of electrode placement for successful EGG recording. These electrodes are then attached to an isolated patient interface and the EGG signals are processed and displayed on a dedicated PC / Laptop.
Shaving of these areas can be required if a subject is too hairy! Once the electrodes are applied, they need 5-10 minutes to settle and form a low impedance contact with the skin. After this period, the subject is placed in a semi recumbent position in a quiet room and asked to relax for 10-15 minutes whilst technical checks are made to ensure a noise free signal is being acquired and baseline resting state ICC pacemaker function is assessed. This first period of recording serves as a control, establishing the normal resting baseline of myoelectrical activity.
The subject is then returned to the upright position and asked to perform a water load test (WLT) which is a validated test of gastric accommodation and activation. The subject is given room temperature water in measured amounts and asked to drink as much water as they can over a 5-minute period until they reach a satiety level of around 7/10 on a visual analogue scale. A value of <300ml indicates poor gastric accommodation, and most subjects tolerate around 800ml comfortably. The subject is then placed back into a semi recumbent position and EGG activity is then recorded for a further 30-minutes. This post stimulation period tells you whether the subject has a normal number of ICC cells or has signs of depleted ICC’s indicative of neuropathy or other dysfunction of the neuromuscular apparatus. A respiration belt is place around the subject’s chest during the test so respiration artefact can be filtered out of the signalling.
These EGG data are displayed in two ways, as power spectral graphs showing the peaks of myoelectrical activity at different time points and frequencies during the entire test and, perhaps more easy to interpret, normative graphs which plot the patients responses onto normal range line-plots to help determine subsets of myoelectrical response to stimulation.
Subsets of EGG response:
There are three basic subsets based upon the three cycle per minute gastric myoelectrical activity (GMA) obtained from EGG recordings when comparing with healthy controls:
1 – Normal study – Normal baseline 3cpm GMA activity and normal response to water load
2 – Hypernormal Study – Normal Baseline 3cpm GMA activity and enhanced response to water load test – indicative of pyloric outflow obstruction – usually responds to pyloric dilatation, for example.
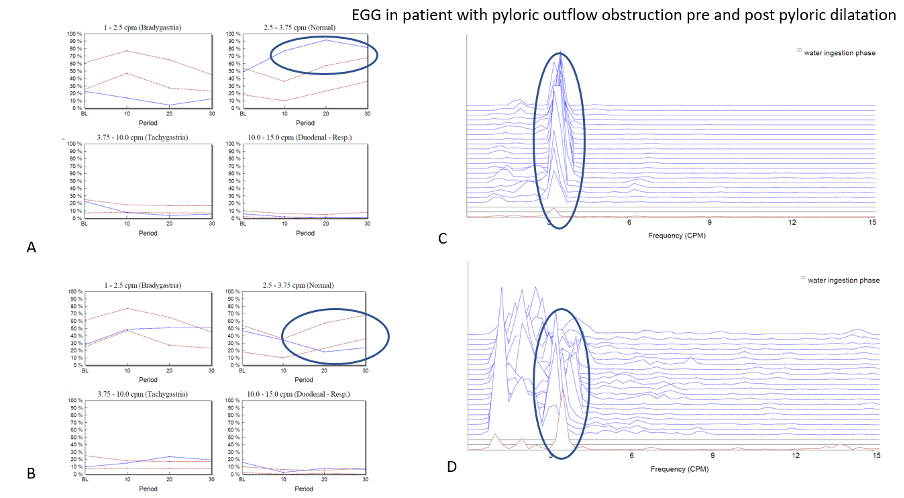
Figure 2 – Panel A shows normative graphs for the four different frequency bands interrogated on the patient’s first visit. In this example it can be seen that at baseline, the normal 3cpm GMA is within the normal range but after the WLT this activity increases strongly to above the normal range indicating a hypernormal profile. This can be thought of as akin to a hypercontractile distal oesophageal contraction which is often observed when a patient has a degree of gastro-oesophageal outflow obstruction. This led to the patients having a pyloric dilatation and the study was repeated within 2-weeks of the procedure. The graphs seen in panel B shows that the patients EGG response to the WLT has now completely resolved as the pyloric outflow obstruction has been successfully treated. Panels C and D power spectral graphs of the EGG throughout the study at baseline (red) and following WLT (blue).
3 – Hyponormal Study – Reduced baseline 3cpm GMA activity and reduced response to water load – indicative of depletion of ICC’s / neuropathy – typical diabetic type pattern which often responds poorly to medical treatment and often requires nutritional intervention.
There are two more interesting subsets that are commonly seen:
4 – Functional Dyspeptic profile – Normal baseline 3cpm GMA activity and increased frequency (tachygastric) response to water load (with or without normal gastric accommodation) – This profile represents mostly normal but disordered neural function that is most commonly associated with symptoms such as nausea and early satiety and can often respond well to prokinetic therapy.
5 – GORD profile – Normal baseline 3cpm GMA activity and reduced response to water load – indicative of ‘venting’ of gastric contents after the water load test which reduces the stomachs’ ability to empty effectively and which can be often corrected by repairing the hiatal defect and/or reflux.
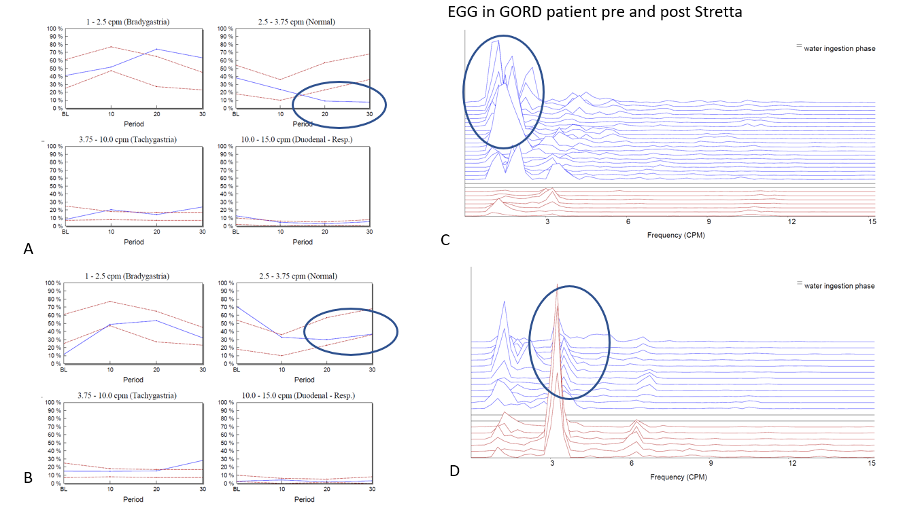
Figure 3 – Panel A shows normative graphs for the four different frequency bands interrogated on the patient’s first visit. In this example it can be seen that at baseline, the normal 3cpm GMA is within the normal range but after the WLT this activity decreases. This is due to the lower oesophageal sphincter yielding at lower pressures than normal allowing reflux and ‘venting’ of intra-gastric pressure. This led to the patient having a STRETTA procedure to improve LOS function and the study was repeated with a couple of months of the procedure. The graphs seen in panel B shows that the patients EGG response to the WLT has now completely normalized as the LOS has improved its ability to reduce reflux. Panels C and D power spectral graphs of the EGG throughout the study at baseline (red) and following WLT (blue).
The novelty of this approach to EGG is that it has allowed for relatively straight forward sub-classification of patients based on known physiological functions to be realized for the first time using objective data (EGG). This has been followed up by outcome studies which have shown that patients sub-classified in this way respond well to directed therapies and that these therapies can often normalize function to an extent.
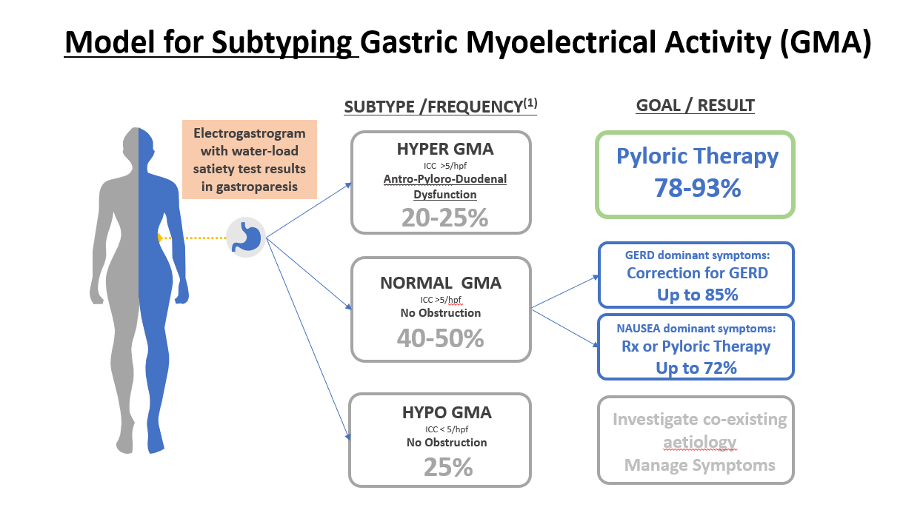
Figure 4 – Shows a treatment model based on US outcomes data that shows how EGG can be used to better direct therapeutic options and improve patient outcomes.
Summary:
EGG has progressed sufficiently to now offer a potentially inexpensive and non-invasive diagnostic test of gastro-pyloric dysfunction. These data have good correlations with known physiological functions and correlate well with known aetiological dysfunctions (such as pyloric outflow obstruction and GORD). In addition, identification of these dysfunctions prior to instigating treatment may change management strategies and also improve the outcome for patients by implementing complementary therapeutic approaches. Just as high resolution oesophageal manometry with the addition of impedance has become an indispensable tool to our diagnostic armory in GI Physiology units, EGG in combination with gastric emptying has a newly established potential to reveal additional important physiological data in patients with complex foregut dysfunction, from functional dyspepsia to gastroparesis.
Acknowledgements – Thanks to Dr Mark Noar from 3CPM for providing input into this text and providing the EGG examples of pre and post treatment responses.
References
1. Stanghellini, V., Chan, F.K., Hasler, W.L., Malagelada, J.R., Suzuki, H., Tack, J., Talley, N.J.: Gastroduodenal Disorders. Gastroenterology 150(6), 1380-1392 (2016). doi:10.1053/j.gastro.2016.02.011
2. Pasricha, P.J., Grover, M., Yates, K.P., Abell, T.L., Bernard, C.E., Koch, K.L., McCallum, R.W., Sarosiek, I., Kuo, B., Bulat, R., Chen, J., Shulman, R.J., Lee, L., Tonascia, J., Miriel, L.A., Hamilton, F., Farrugia, G., Parkman, H.P.: Functional Dyspepsia and Gastroparesis in Tertiary Care are Interchangeable Syndromes With Common Clinical and Pathologic Features. Gastroenterology 160(6), 2006-2017 (2021). doi:10.1053/j.gastro.2021.01.230
3. Tack, J., Schol, J., Horowitz, M.: Gastroparesis: A Dead-end Street After All? Gastroenterology 160(6), 1931-1933 (2021). doi:10.1053/j.gastro.2021.02.042
4. Chokshi, R.V., Chang, L.: Is It Time to Abandon Gastric Emptying in Patients With Symptoms of Gastroparesis and Functional Dyspepsia? Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association (2021). doi:10.1016/j.cgh.2021.05.031
5. Huizinga, J., Chen, J., Mikkelsen, H., Wang, X.-Y., Parsons, S., Zhu, Y.: Interstitial cells of Cajal, from structure to function. Frontiers in Neuroscience 7, 43 (2013).
6. Bashashati, M., McCallum, R.W.: Is Interstitial Cells of Cajal‒opathy Present in Gastroparesis? J Neurogastroenterol Motil 21(4), 486-493 (2015). doi:10.5056/jnm15075
7. Jieyun, Y., Jiande, D.Z.C.: Electrogastrography: Methodology, Validation and Applications. J Neurogastroenterol Motil 19(1), 5-17 (2013). doi:10.5056/jnm.2013.19.1.5
8. Varghese, C., Carson, D.A., Bhat, S., Hayes, T.C.L., Gharibans, A.A., Andrews, C.N., O’Grady, G.: Clinical associations of functional dyspepsia with gastric dysrhythmia on electrogastrography: A comprehensive systematic review and meta-analysis. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society, e14151 (2021). doi:10.1111/nmo.14151
9. Leahy, A., Besherdas, K., Clayman, C., Mason, I., Epstein, O.: Abnormalities of the electrogastrogram in functional gastrointestinal disorders. The American journal of gastroenterology 94(4), 1023-1028 (1999). doi:10.1111/j.1572-0241.1999.01007.x
10. Wellington, J., Scott, B., Kundu, S., Stuart, P., Koch, K.L.: Effect of endoscopic pyloric therapies for patients with nausea and vomiting and functional obstructive gastroparesis. Autonomic neuroscience : basic & clinical 202, 56-61 (2017). doi:10.1016/j.autneu.2016.07.004
11. Wellington, J., Stuart, P., Westcott, C., Koch, K.L.: Obstructive Gastroparesis: Patient Selection and Effect of Laparoscopic Pyloroplasty. Journal of Gastrointestinal Surgery 24(8), 1778-1784 (2020). doi:10.1007/s11605-019-04240-x
12. Ye, Y., Jiang, B., Manne, S., Moses, P.L., Almansa, C., Bennett, D., Dolin, P., Ford, A.C.: Epidemiology and outcomes of gastroparesis, as documented in general practice records, in the United Kingdom. Gut 70(4), 644 (2021). doi:10.1136/gutjnl-2020-321277
13. Lin, Z., Sarosiek, I., Forster, J., Damjanov, I., Hou, Q., McCallum, R.W.: Association of the status of interstitial cells of Cajal and electrogastrogram parameters, gastric emptying and symptoms in patients with gastroparesis. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society 22(1), 56-61, e10 (2010). doi:10.1111/j.1365-2982.2009.01365.x
14. Mancilla Asencio, C., Gálvez-Arévalo, L.R., Tobar Almonacid, E., Landskron-Ramos, G., Madrid-Silva, A.M.: Evaluation of gastric motility through surface electrogastrography in critically ill septic patients. Comparison of metoclopramide and domperidone effects: A pilot randomized clinical trial. Revista de Gastroenterología de México (English Edition) 84(2), 149-157 (2019). doi:https://doi.org/10.1016/j.rgmxen.2018.06.009
15. Chen, J.D., Lin, Z.Y., Edmunds, M.C., 3rd, McCallum, R.W.: Effects of octreotide and erythromycin on gastric myoelectrical and motor activities in patients with gastroparesis. Digestive diseases and sciences 43(1), 80-89 (1998). doi:10.1023/a:1018876021156
16. Tack, J., Janssen, P., Masaoka, T., Farré, R., Van Oudenhove, L.: Efficacy of buspirone, a fundus-relaxing drug, in patients with functional dyspepsia. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association 10(11), 1239-1245 (2012). doi:10.1016/j.cgh.2012.06.036

